彩色细胞编码技术:可分析肿瘤单细胞
| 导读 |
想要获得更好的癌症诊断和治疗,有可能取决于分子水平上更好地认识单细胞的能力。一项新的研究提供了一种更全面的方法分析单细胞的独特行为,利用一系列颜色来显示模式,揭示出一个细胞是否会发生癌变及其原因。
来自华盛顿大学的一个研究小组开发了一种彩色编码细胞新方法,以此阐明了100种生物标志物,在当前研究标准之上提高了10倍,帮助分析了来自培养物或组织... |

想要获得更好的癌症诊断和治疗,有可能取决于分子水平上更好地认识单细胞的能力。一项新的研究提供了一种更全面的方法分析单细胞的独特行为,利用一系列颜色来显示模式,揭示出一个细胞是否会发生癌变及其原因。
来自华盛顿大学的一个研究小组开发了一种彩色编码细胞新方法,以此阐明了100种生物标志物,在当前研究标准之上提高了10倍,帮助分析了来自培养物或组织活检的单个细胞。这一研究工作发表在本周的《自然通讯》(Nature Communications)杂志上。
领导这一研究的是华盛顿大学生物工程学教授高虓虎(Xiaohu Gao)博士。其科研兴趣主要集中于纳米科技和生物科技在临床医学中的应用,并在光学条形码技术, 活体医学成像, 多功能纳米材料等领域作出过突出贡献。其中在Nature Nanotechnology (IF 20.57), Nature Biotechnology (IF 22.29), Lancet Oncology (IF 13.28)等国际顶级杂志上发表文章数十篇,单篇论文最高引用次数超过1,000次,还有多篇文章单篇他引次数过百。
高虓虎说:“发现这一过程是该领域一项前所未有的突破。这一技术为单细胞分析和临床诊断开辟了令人兴奋的机会。”
当前的方法是采用少量的颜色指出细胞的生物标记(能够表明特殊,及潜在异常或病变细胞的特征)。新研究是建立在这些方法的基础之上,科学家们能够对大量的生物标记进行测试,随后根据这些测试显示出的模式来了解一个细胞的特性。
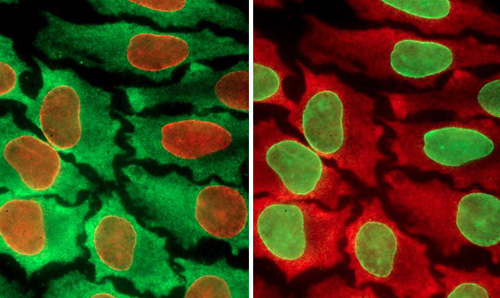
同一样品经过不同的彩色编码处理
华盛顿大学研究小组创建了一个循环过程,使得科学家们能够在单个细胞中测试多达100种生物标记。而在此之前,研究人员一次只能测试10种。这一分析利用了量子点(quantum dots)技术。量子点是一些半导体材料荧光球,它是许多电子设备,包括智能电话和收音机中材料的缩小版本。这些量子点的直径为2-6纳米,根据大小它们会发出不同的颜色。尽管许多量子点论文曾经尝试扩大单细胞测试中的生物标志数量,此前还未做过周期性测试。这种方法实质上重复利用了相同的组织样本,一轮检测10个为一组的生物标记。高虓虎说:“蛋白质是细胞功能和行为的基础构件,然而它们在细胞内的组成非常复杂。你需要观察一些指标(生物标记)来了解正在发生的事情。”
在新研究中,高虓虎和研究小组购买了一些抗体,这些抗体已知与他们希望在细胞中测试的特异性生物标记结合。他们在一个液体溶液中将量子点与抗体偶联,然后注入到一个组织样本中。随后,他们利用显微镜寻找细胞中的荧光颜色。如果他们在组织样本中看到特异的量子点颜色,他们就知道细胞中存在哪些对应的生物标记。
在完成一个循环后,高虓虎和华盛顿大学生物工程学博士生、论文的共同作者Zrazhevskiy一起,将一种低pH液体注入到细胞组织中,中和彩色荧光,实质上是为下一轮进行样本清理。值得注意的是,甚至在10个这样的循环后组织样本也不会降解。
对于癌症研究和治疗,能够以高分辨率观测单个细胞检测它的细节尤其重要。例如,如果人体内99%的癌细胞对某一治疗药物产生反应,1%的不产生反应,分析和了解反应不同的1%细胞的分子构成具有重要的意义。
“当你测试一些有前景的药物时,仍有一些细胞通常不会对治疗产生反应。它们看起来是一样的,而你没有工具去观测它们的蛋白质构件。这一技术将有助于我们开发出新药物和治疗方法,”高虓虎说。这一过程成本相对较低且简易,高虓虎希望可以将这一程序自动化。他设想该设备应该有一个容纳组织样本的暗槽,利用细拉丝泵在循环间注入和真空吸出液体。暗槽的下方有一个显微镜能够在每个阶段拍摄照片。所有的图像都能在计算机上定量,科学家们和医生们可以在计算机上观察颜色的强度和范围。

量子点(quantum dots)技术
高虓虎希望能与其他公司和研究人员展开协作,推动自动化进程和临床应用。他说:“技术已准备就绪。既然已将它开发出来,我们将为其临床应用做准备,特别是在系统生物学、肿瘤学和病理学等领域。”
高虓虎教授介绍
高虓虎教授感受科学前沿,在生物医药纳米工程,分子工程,分子成像及分子诊断等多个领域从事科学研究。以量子点(quantum dots QD)科学为研究核心,通过对量子点功能性和纳米结构深入探讨研究超灵敏的金属纳米粒检测技术。利用量子点光学性质,发展一种高通量光学“条形码”,用于基因、蛋白质、细胞等生物分子检测;根据个体癌症病例特异性特点,开发一种多色彩共价量子点技术,原位定量形成肿瘤标记物。系统研究多功能性纳米探针,用于目视观察其在活性细胞中的分子动态,用于早期癌症和心血管疾病的诊断;无机纳米粒和高分子材料有机结合作为小分子药物分子,siRNA和DNAs等治疗分子的药物载体,研究其功能性和靶向性行为。
原文链接
Quantum dot imaging platform for single-cell molecular profiling
Study of normal cell physiology and disease pathogenesis heavily relies on untangling the complexity of intracellular molecular mechanisms and pathways. To achieve this goal, comprehensive molecular profiling of individual cells within the context of microenvironment is required. Here we report the development of a multicolour multicycle in situ imaging technology capable of creating detailed quantitative molecular profiles for individual cells at the resolution of optical imaging. A library of stoichiometric fluorescent probes is prepared by linking target-specific antibodies to a universal quantum dot-based platform via protein A in a quick and simple procedure. Surprisingly, despite the potential for multivalent binding between protein A and antibody and the intermediate affinity of this non-covalent bond, fully assembled probes do not aggregate or exchange antibodies, facilitating highly multiplexed parallel staining. This single-cell molecular profiling technology is expected to open new opportunities in systems biology, gene expression studies, signalling pathway analysis and molecular diagnostics.
Supersandwich Cytosensor for Selective and Ultrasensitive Detection of Cancer Cells Using Aptamer-DNA Concatamer-Quantum Dots Probes.
In this work, a signal amplification supersandwich strategy was developed for highly selective and sensitive detection of cancer cells using aptamer-DNA concatamer-quantum dots (QDs) probes. First of all, electrode materials denoted as MWCNTs@PDA@AuNPs were fabricated by multiwall carbon nanotubes (MWCNTs), gold nanoparticles (AuNPs), and polydopamine (PDA) using a layer-by-layer technique. Then, the prepared bases as matrices were applied to bind concanavalin A (Con A), resulting in high stability, bioactivity, and capability for cell capture. Meanwhile, aptamer-DNA concatamer-QDs were designed via DNA hybridization followed by covalent assembling, which incorporated the specific recognition of the aptamer with the signal amplification of the DNA concatamer and QDs. With aptamer-DNA concatamer-QDs as recognizing probes, the model cancer cells (CCRF-CEM cells) were detected using a MWCNTs@PDA@AuNPs modified electrode with trapped Con A by means of fluorescence and electrochemical methods. The proposed supersandwich cytosensor showed high sensitivity with the detection limit of 50 cells mL-1. More importantly, it could distinguish cancer cells from normal cells, which indicated the promising applications of our method in clinical diagnosis and treatment of cancers.
A universal quantum dots-aptamer probe for efficient cancer detection and targeted imaging.
Targeted quantum dots have shown as an analytical and imaging tool for cancer detection and molecular imaging. Aptamers have recently been demonstrated as ideal candidates for molecular targeting applications. In the present work, quantum dots (QDs) were encapsulated with functional poly(ethylene glycol)-phospholipids to improve their solubility in water solution. The as-prepared QDs were then conjugated with the AS1411 aptamer recognizing the nucleolin overexpressed on cancer cell surface. The recognition of QD-Aptamer nanocomplex to breast cancer cells was demonstrated using confocal microscopy, and the viability of QDs-aptamer bioconjugate bound cells were not affected within 24 h, indicating that the probe was biocompatible and suitable for in vitro diagnostic assays and live cell imaging. Such well-defined aptamer-conjugated QDs nanoprobe was simple and universal to be extended to prepare various aptamer-nanoparticles hybrid systems for cancer targeting and molecular imaging applications.
Assessing breast cancer margins ex vivo using aqueous quantum-dot-molecular probes.
Positive margins have been a critical issue that hinders the success of breast- conserving surgery. The incidence of positive margins is estimated to range from 20% to as high as 60%. Currently, there is no effective intraoperative method for margin assessment. It would be desirable if there is a rapid and reliable breast cancer margin assessment tool in the operating room so that further surgery can be continued if necessary to reduce re-excision rate. In this study, we seek to develop a sensitive and specific molecular probe to help surgeons assess if the surgical margin is clean. The molecular probe consists of the unique aqueous quantum dots developed in our laboratory conjugated with antibodies specific to breast cancer markers such as Tn-antigen. Excised tumors from tumor-bearing nude mice were used to demonstrate the method. AQD-Tn mAb probe proved to be sensitive and specific to identify cancer area quantitatively without being affected by the heterogeneity of the tissue. The integrity of the surgical specimen was not affected by the AQD treatment. Furthermore, AQD-Tn mAb method could determine margin status within 30 minutes of tumor excision, indicating its potential as an accurate intraoperative margin assessment method.
来源:生物探索
 腾讯登录
腾讯登录
还没有人评论,赶快抢个沙发